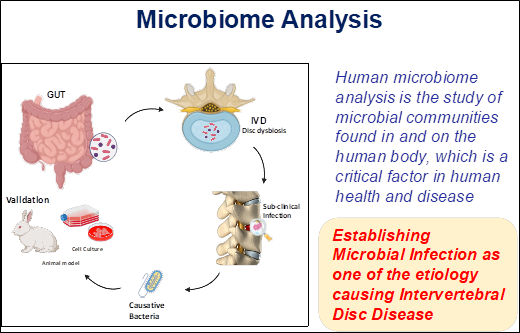
One of our primary hypotheses is that sub-clinical infection may be a driving factor in intervertebral disc (IVD) degeneration. The human microbiome—a complex ecosystem of trillions of bacteria, fungi, and viruses—is indispensable to our health. It supports digestion, synthesizes essential vitamins, and bolsters the immune system against pathogens. However, when this delicate balance is disrupted, a state known as dysbiosis occurs. Dysbiosis is increasingly linked to systemic conditions, including inflammatory bowel disease (IBD), type 2 diabetes, and metabolic disorders.
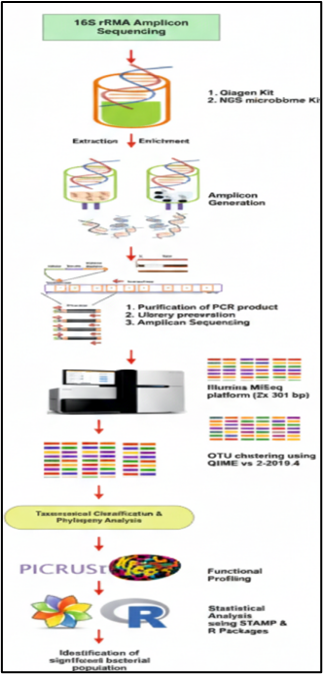
To investigate these connections, we utilize metagenomic analysis. This advanced approach has revolutionized our understanding of microbial life, offering a high-resolution view of genetic and functional diversity that traditional culturing methods cannot achieve. Our recent findings suggest that gut dysbiosis may facilitate sub-clinical infections within the intervertebral disc, opening a groundbreaking research avenue for treating chronic low back pain. By maintaining a diverse microbiome—supported by a nutrient-rich diet and a healthy lifestyle—we can foster a symbiotic relationship between our microbes and the immune system, potentially mitigating the root causes of IVD degeneration.
Genomics-Driven Research into IVD Degeneration
The genomics lab is established to unravel the molecular mechanism underlying in the IVD degeneration, which causes low back pain. The analysis of genes involved in various functions and their pathways been studied with the help expression and functional analysis. Also further studying the metagenome of IVD. Here the researchers work on the disc tissue of IVD from patients and control. Currently the research aims to elucidate the IVD microbiome using next generation sequencing platforms and the leads into tranlsational therapeutics to mitigate the onset of IVDD.
IVD Metagenomics and Subclinical Infection
By using high-throughput genomics approaches, including PCR and NGS, we have successfully identified a distinct microbiota present within IVD tissues in both healthy individuals and patients. Our metagenomic studies highlight a significant shift in the abundance of commensal versus pathogenic bacteria in degenerated discs.
Further, we have documented the evidence of "dysbiosis"—characterized by altered bacterial ratios across various disease phenotypes. These findings have profound implications for our understanding of the etiology of subclinical infections in the IVDD. By defining these microbial signatures, we are opening new avenues for non-surgical, targeted therapeutic interventions. In our ongoing research we are validating the findings using in vitro (primary nucleus pulposus cells) and in vivo (rabbit) models to identify the prime causative microbes for disc degeneration and proving its role.
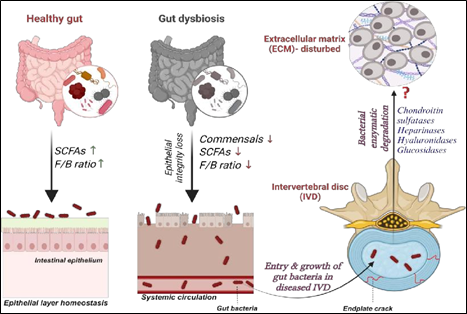
Gut-Spine Axis:
Gut microbiome being the important contributors of inflammatory disorders, our research explores its specific role in disc degeneration and pain severity. Variation in abundance of gut microbiome in disc degeneration patients with respect to healthy volunteers is assessed. Understanding their role in dysbiosis which leads to disease severity in IVDD patients is explored using NGS-16S metagenome approach. Our findings revealed that gut biome dysbiosis contributes to the severity of disc degeneration and pain sensation. These results establishes the existence of a “Gut-Spine axis” and our ongoing study seek to define the casual relationship between gut-derived microbes and the progression of IVDD.